Ozone is a highly reactive gas that when inhaled can cause burning and irritation of the lining of the lungs. This is particularly harmful to individuals with breathing problems like asthma, as well as for people who work outside when ozone levels are high.
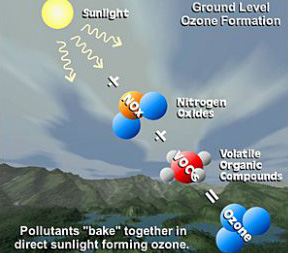
Ozone is not directly emitted by human activities, but is formed when emissions of nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the atmosphere in sunlight and heat. Since sunlight and heat are an important factor in its formation, ozone is usually a summertime pollutant of concern in urban areas, but under very unique conditions ozone can form in the wintertime as is the case in Utah’s Uinta Basin.
WATCH: Utah’s Summertime Ozone (1:28)
What is a State Implementation Plan?
Certain areas in Utah have ozone pollution levels that are too high, exceeding national health standards. Addressing this pollution requires specific actions and rules tailored to each affected area.
Areas that are not meeting the National Ambient Air Quality Standard for ozone are required to implement policies and regulations that aim to reduce NOx and VOC emissions in an attempt to limit ozone formation. The current national standard was set in 2015 at 70 parts per billion (ppb). Areas not meeting this standard are called nonattainment areas, and are required to implement emission reduction policies as part of a State Implementation Plan (SIP).
A SIP is an all-inclusive document that overviews everything the state is doing to address pollution and shows that it is complying with all applicable statutory elements of the Clean Air Act. The state of Utah is required to develop and implement a SIP for each nonattainment area, and additional SIPs each time an area is reclassified to a more stringent status. Once adopted, the policies and programs contained within the SIP have the effect of law, and can be enforced accordingly.
The elements of a SIP can include things like:
- Reasonably Available Control Technologies
- Reasonably Available Control Measures
- Motor Vehicle Inspection and Maintenance (I/M) Programs
- Emission Inventories
- Modeled Attainment Demonstrations
However, each pollutant regulated under the Clean Air Act has its own unique set of requirements, which can also change from one nonattainment status to another. Therefore, each SIP adopted by the state is unique and comes with its own individual requirements.
Northern Wasatch Front Nonattainment Area
The Northern Wasatch Front nonattainment area includes all of Salt Lake and Davis counties, as well as portions of Weber and Tooele counties. The area was designated as a marginal nonattainment area in 2018. The area failed to attain the standard by the marginal attainment date and was further reclassified to a moderate nonattainment area in October, 2022. As a result, the state of Utah developed and submitted a SIP to address moderate nonattainment requirements. The state of Utah is now in the process of developing a serious nonattainment SIP in anticipation of a potential redesignation, and in efforts to continue to reduce emissions and improve air quality.
View the technical supporting documents for Northern Wasatch Front SIPs:

Uinta Basin Nonattainment Area
The Unita Basin nonattainment area includes portions of Duchesne and Uintah counties in the north eastern portion of the state. The area was designated as a marginal nonattainment area in 2018. The area has been granted several timeline extensions by the EPA to demonstrate attainment of the standard, and as a result is currently designated as a marginal nonattainment area. More detailed information on the Uinta Basin regulatory status and history can be found here at Uinta Basin Ozone.

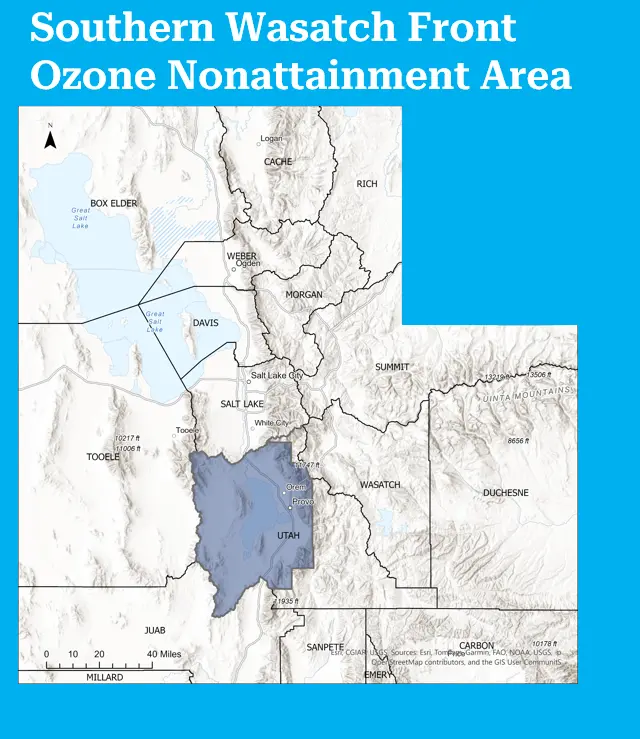
Southern Wasatch Front Nonattainment Area
In August 2018, the EPA designated the Southern Wasatch Front as a nonattainment area for ozone based on the 2015 National Ambient Air Quality Standard. This area includes Utah County, and borders the Northern Wasatch Front. The Southern Wasatch Front attained the standard by the marginal attainment date of August 3, 2021. As a result, the state of Utah is not required to develop or implement any additional SIPs for this area, and the state will be working towards submitting a maintenance plan for the area in the coming years in an effort to move the area out of a nonattainment designation.